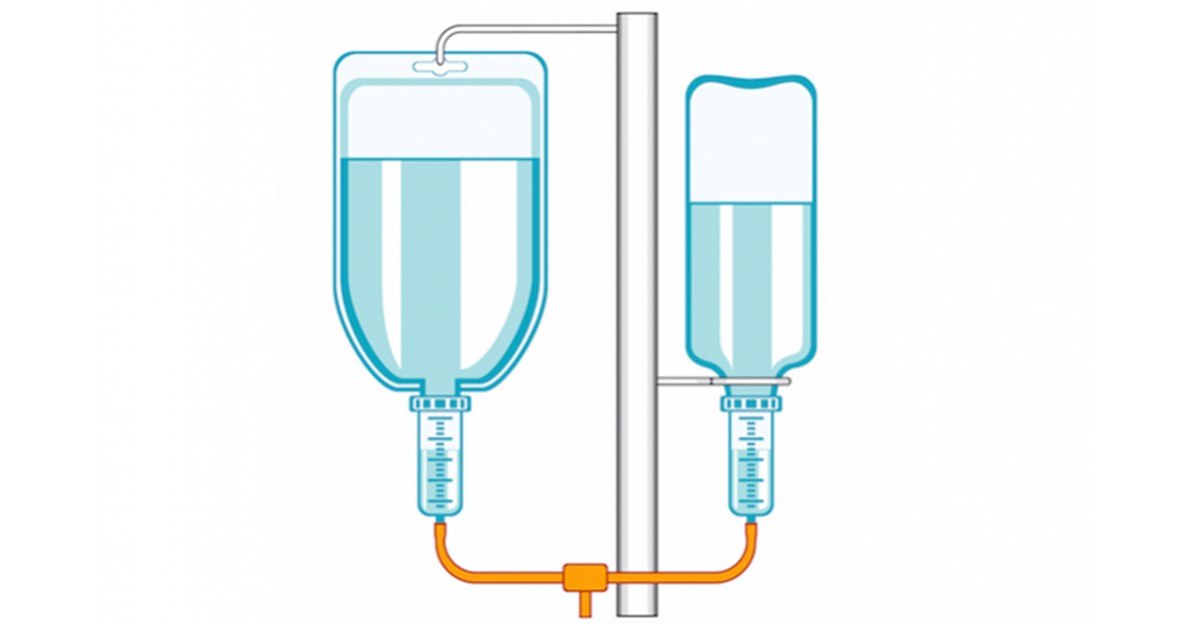
Different types of IV fluids (Intravenous fluids) or drip helps to restore the normal fluid volume or electrolyte balance in situations where oral therapy cannot be effective. Various IV fluids are required in different unique situations in clinical settings.
About 60% of an adult and 80% of a neonate body contains water. This amount varies based on age, sex, and body mass. Different types of IV fluids move in two main compartments. Extracellular fluids occupy one-third, and the intracellular fluid takes two-thirds of the body. Intracellular fluids move inside the cell.
The extracellular fluids move outside the cell in the interstitial spaces (between the blood vessels), intravascular fluids (blood vessels), and transcellular fluids (cerebrospinal, synovial, pleural, and peritoneal fluids)The movement of these fluids across the membrane is by osmosis.
Most patients who are admitted into the hospital do receive different types of IV fluids.
Different types of IV fluids
There are three main different types of IV fluids available. They are:
- Crystalloids
- Colloids
- Blood products, and blood.
Crystalloids are the most used type of IV fluids, and we will discuss it extensively.
Crystalloids
Crystalloids are solutions of water with electrolytes and can pass across semi-permeable membranes easily from the bloodstream into the cells, tissues. Crystalloids move rapidly from the intravascular space to the interstitial cells.
They stay in the extracellular cells for a long time, which means large volumes than colloids are needed for resuscitation. In the end, water from the colloids diffuses through intracellular fluids. Crystalloids are differentiated by tonicity in relation to the plasma. Tonicity is the measure of the effective osmolarity in the cell.
According to tonicity, solutions are classified into three main types:
- Isotonic,
- hypertonic, and
- hypotonic solutions.
Isotonic Solutions
Isotonic solutions have the same osmotic pressure as that of the body fluids such as blood and tears. The value corresponds to 0.9% NaCl or dextrose aqueous solution, which is the measure of isotonicity.
The solution has a concentration of dissolved particles similar to plasma, and osmolarity of about 280 to 300 mOsm/litre just as the body. Electrolytes with a value of about 310 mEq/L are considered isotonic.
They stay in the extracellular spaces and distribute to the intravascular compartment – interstitial spaces (tissues) and intravascular fluids (blood vessels) increasing their volume. It maintains the balance between the two spaces.
Isotonic solutions must not cause tissue swelling, contraction, or any form of discomfort to any of the body tissue.
Examples of isotonic fluids include Dextrose 5% in water, 0.9% Sodium Chloride (Normal Saline), Ringers Lactate (Hartmann).
Normal Saline (0.9% Sodium Chloride)
An isotonic and crystalloid solution. It has an osmolarity of 308 mOsm/L. It contains water, and two electrolytes sodium and chloride (both have 154 mEq/L electrolyte content). Sodium is the major cation in the extracellular fluids, while chloride is the major anion in the extracellular fluids.
Normal saline does not enter the intracellular fluids, making it the best fluid to correct extracellular fluid volume deficit.
Uses of Normal Saline: The most used types of IV fluids. Used in diabetic ketoacidosis, hyponatraemia in trauma, burns, blood transfusion, resuscitation, shock, supplement in blood products administration.
Precaution: Used with caution in patients with oedema, heart failure, kidney complications due to volume overload resulting from sodium retention.
Normosol™-R
Normosol and NaCl are similar types of IV fluids. This isotonic solution has an advantage over normal saline in sepsis resuscitation because it lowers chloride levels, and also reduces acidosis.
It contains sodium chloride, sodium acetate, potassium chloride, magnesium chloride, sodium gluconate, and hexahydrate. It is used in the replacement of acute losses of extracellular fluid.
Precaution: Used with caution in patients with congestive heart failure, renal insufficiency, and oedema. Also, patients with metabolic acidosis, hyperkalaemia.
Dextrose 5% in water (D5W)
Isotonic crystalloid solution. Physiologically, when dextrose is metabolized, it provides free water and becomes a hypotonic solution. It has an osmolarity of 252 mOsm/L. It also expands both intracellular and extracellular fluids.
Uses of Dextrose 5% in water: A litre of 5% Dextrose in water contains 50g of glucose, hence it can be used to supply energy. It is used to raise the total fluid volume by supplying water, and to correct the increase in osmolarity of body fluids.
Precaution: It is never used for resuscitation due to the risk of hyperglycaemia or osmotic diuresis. Used with caution in renal, cardiac patients. Can cause cranial oedema.
Ringer’s Lactate (Hartmann’s)
*Ringers Lactate should not be confused with Ringer’s solution. They have similar content and indication, but the ringer’s solution has no lactate. It is better when lactate is not metabolized, especially in liver diseases.
Ringers lactate or Lactated Ringer’s 5% Dextrose in Water (D5LRS) is an isotonic crystalloid solution. It provides an almost balanced number of electrolytes to the body – potassium (4 mEq/L), sodium (130 mEq/L), calcium (3 mEq/L), and chloride (109 mEq/L). Hence, the composition is close to that of the body fluid.
Sodium, potassium, calcium are major cations in the extracellular fluids (ECF), while chloride is a major anion in the ECF. However, the ringer’s lactate has no magnesium or energy (calories).
Uses of Ringers Lactate: Ringers lactate has a bicarbonate precursor to help prevent metabolic acidosis. It is used in dehydration, hyponatraemia, burns, GIT fluid loss, acute blood loss, hypovolemia due to third spacing (when fluids move from intravascular cells into interstitial spaces – the non-functional areas).
It is also the choice of resuscitation in some patients.
Precaution: Not to be used in renal failure due to potassium content. Lactate cannot be metabolized (converted to bicarbonate) by those with liver diseases, or lactic acidosis. It should also be avoided in such conditions.
Hypertonic Solutions
The solution has higher osmotic pressure than the normal body fluid (0.9% NaCl) hence they draw water from the cells and interstitial tissues into the extracellular compartment, causing an increase in their volume. This might shrink the cells. They have an osmolarity of >300 mOsm/litre.
These types of IV fluids are volume expanders since they draw water from intracellular cells to ECF.
Mannitol Solution
Available in both 10% and 20% strength. It is made of simple sugar (6-carbon) dissolved in water. Mannitol has an osmolarity of 549 mOsm/l. It is an effective osmotic diuretic. Mannitol does not move away from intravascular spaces to the interstitial spaces, but remains confined.
Uses of mannitol solution:
- Enhance diuresis in treating the oliguric phase of acute kidney failure before progression to irreversible kidney failure.
- Reduces intracranial pressure and cerebral oedema.
- Also used to reduce intraocular pressure in glaucoma.
- In poisoning to reduce renally excreted toxins.
Precaution: Severe dehydration, internal bleeding, anuria due to severe renal disease, pulmonary oedema, congestion.
Hypertonic Sodium Chloride IV Fluids
A hypertonic crystalloid solution. It has a NaCl concentration that is higher than the plasma, hence fluids move from intracellular cells to the ECF. This solution is infused slowly to avoid excess volume in the ECF, circulatory overload, pulmonary oedema.
Available as 5% NaCl, and 3% NaCl strengths. The 3% form has NaCl content of 513 mEq/L, while the 5% has NaCl content of 855 mEq/L with an osmolality of 1030 mOsm/L, and 1710 mOsm/L, respectively.
Uses: Used in critical conditions to treat acute hyponatraemia.
Hypertonic Dextrose Solutions
This hypertonic crystalloid solution contains 5% dextrose. Though it is a bit hypertonic, dextrose is metabolized, making it isotonic in the body.
Use: To provide kilocalorie in short-term management.
Dextrose 5% in ½ Normal Saline (D5 ½ NS)
A hypertonic solution of 5% dextrose in 0.45% normal saline. It has an osmolality of 406 mOsm/L. It is a common postoperative fluid.
Uses: Used later in diabetic ketoacidosis after starting with normal saline and half normal saline. Used when the blood sugar falls below 250 mg/DL.
Dextrose 5% in Normal Saline
It is a hypertonic solution. It has 5% dextrose in 0.9% normal saline and an osmolarity of 560 mOsm/L.
Used in Addison’s crisis, temporary treatment of shock, or circulatory insufficiency.
Precaution: Contraindicated in renal and cardiac diseases.
Dextrose 10% in Water (D10W)
This is a hypertonic solution used to provide water, energy (380 kcal/L), to treat ketosis of starvation. It does not have any electrolytes.
Precaution: A central line should be used for the infusion. Also, avoid using the same line used for blood products, as red blood cell hemolysis may occur.
Dextrose 20% in Water (D20W)
A hypertonic solution, and an effective osmotic diuretic.
Dextrose 50% in Water (D50W)
A hypertonic solution that is administered through rapid IV bolus in hypoglycaemia.
Dextrose 5% in Ringers Lactate
The hypertonic solution has an osmolarity of 575 mOsm/L. It is used to supply water, electrolytes (Na, K, Ca, Cl), and energy. It is also an alkalizing agent.
Precaution: Dextrose 5% in Ringers Lactate should not to be used in newborns (<28 days) to avoid ceftriaxone calcium precipitation, causing death. Also avoided in patients that cannot tolerate lactate.
Normosol-M and Dextrose Solution
A hypertonic solution containing balanced electrolytes – sodium, potassium, magnesium, chloride, and bicarbonate in addition to 5% dextrose. It has an osmolarity of 363 mOsmol/litre.
Uses: It can supply the body with water and electrolyte needs. With 170 calories, it can provide small energy.
Precaution: Patients with congestive heart failure, renal insufficiency, pulmonary oedema.
Hypotonic Solution
A hypotonic solution has osmolarity lower than 280 mOsm/litre and electrolyte level less than 250 mEq/L. The concentration of dissolved particles is lower when compared with the plasma, therefore fluids move from ECF to the intracellular fluid to maintain homeostasis. This can cause the cells to swell, and possibly burst.
These types of IV fluids are used to treat dehydration, supply water for the excretion of waste from the body. Examples are 0.45% Sodium Chloride, 2.5% Dextrose in Water (D2.5W)
0.45% Sodium Chloride
A hypotonic solution is also called half strength-normal saline (0.45 NaCl). It has an osmolarity of 154 mOsm/L, and electrolyte content of 77 mEq/L of NaCl.
Use: To treat hypovolemia, hypernatraemia, hyperosmolar conditions. Also used in diabetic ketoacidosis, gastric fluid loss from nasogastric suctioning, or vomiting.
Precaution: Used in caution in patients with heart failure, renal insufficiency.
Other examples of hypotonic fluids are 0.33% Sodium Chloride Solution, used together with dextrose to increase tonicity. Another type, 0.225% Sodium Chloride Solution, is a paediatric solution used together with dextrose as a maintenance fluid.
2.5% Dextrose in Water (D2.5W)
Used to treat low potassium, sodium level. It is also used for dehydration. Do not use the same line used for blood products to prevent red blood cell hemolysis.
Colloids
Colloids are types of IV fluids with large proteins and other solutes that cannot pass through semi-permeable membranes. Hence, they stay in the intravascular spaces, increasing the volume. They maintain high osmotic pressure in the blood, pulling fluids from interstitial and extracellular spaces. They have high molecular weights and are gelatinous.
Colloids are either natural, e.g. albumin or artificial, e.g. hydroxyethyl starches (HES), dextran, and gelatin. They are used for volume expansion.
Albumin (Plasma Protein)
Albumin is the natural colloid with about 60% of all plasma proteins. It undergoes two phases when administered. There are two forms – 5% solutions (isooncotic) and the 25% solution (hyperoncotic). The 25% solution causes more volume expansion of about 400% compared to the 80% by the 5% solution. The expansion by the 20% solution is higher than any other colloids.
Albumin has a better safety profile than other colloids, since it is natural. It is, however, more expensive than other colloid types of IV fluids.
Albumin is used to manage burns, lung injury, liver transplantation, and emergency treatment of shock after plasma loss.
Plasma Protein Fraction (PPF)
Prepared from the human plasma just like the albumin. It is used for plasma expansion but can cause more adverse effects such as hypotension, allergy than plasma. Available as a 5% solution.
Dextran (Polysaccharide)
These are highly branched polysaccharides. They are artificial fluids available as saline or glucose solution. Dextrans are produced by synthesis using the bacterial enzyme dextran sucrase.
There are two types: the low-molecular-weight dextrans or dextran-40 with an average weight of 40,000, and the high-molecular-weight dextran or dextran-70 with the weight of 70,000.
Dextran-70 is the 6% solution, while the dextran-40 is the 10% solution. Dextrans cause higher volume expansion when compared to 5% albumin. However, they cause more anaphylactic reactions.
Gelatins
These types of IV fluids and colloids have high molecular weight, but are lower than dextrans. Gelatins are formed from the hydrolysis of collagen. Examples include geloplasma, haemaccel. There are three main types:
- Urea-crosslinked gelatins,
- Oxypolygelatins,
- Succinylated or modified fluid gelatins.
These types of IV fluids are used to treat hypokalemia, volume preload before anaesthesia.
Hydroxyethyl starches (HES)
Derived from a highly branched starch, the amylopectin molecule. This molecule has been stabilized by a substitution reaction. Examples include elohes, hesteril, hextend, etc. A third-generation of hydroxyethyl starch with more effectiveness called tetrastarch has been developed.
For extensive information on colloids, refer here.
READ ALSO: Different types of cannula sizes, colours, and uses.