Pharma giant, Pfizer has announced the voluntary withdrawal of its hypertensive medication, Accuretic (quinapril HCl/hydrochlorothiazide) and another two generics distributed by Greenstone—(quinapril and hydrochlorothiazide and quinapril HCl/ hydrochlorothiazide)
The medications treat hypertension by lowering blood pressure, and reducing the risk of stroke, myocardial infractions and other cardiovascular events. They have been safely used for over 20 years and still have good benefits to the risk ratio profile.
The reason given by Pfizer for the withdrawal is the presence of a high level of nitrosamine, N-nitroso-quinapril, above the Acceptable Daily Intake (ADI) level.
Although we are exposed to nitrosamines in the environment through water, food, dairy products, vegetables, grilled meat, exposure to the impurities above the acceptable level can increase the risk of cancer.
This effect is a long-term effect, and as such, patients taking the drugs will not witness an immediate cancer risk.
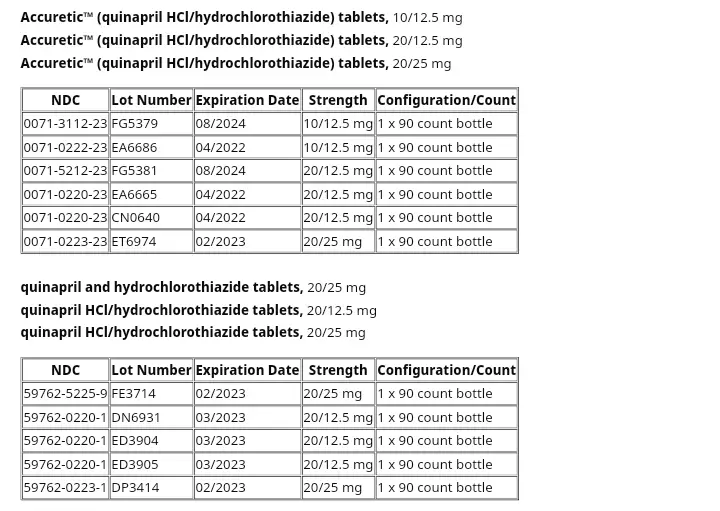
Pfizer is recalling six lots of Accuretic tablets, one lot of quinapril and hydrochlorothiazide tablets, and four lots of quinapril HCl/ hydrochlorothiazide tablets.
They urge all distributors and wholesalers with the affected batches and lots to stop the distribution and quarantine the products immediately. Patients who are placed on the medication are also advised to meet the doctor to ensure they are not using the affected lots.
Here is the list of all the withdrawn lots with the number, expiration, strength, and count.
Source: Pfizer












