One of the many questions patients ask prescribers and pharmacists is the difference between generic vs brand name drugs. It is good to note the differences and similarities between them, to help patients in making medication choices.
However, there are certain conditions that can warrant the prescription of branded medicine or generic medicines, such as certain modified- or controlled-release drugs, drugs with difference in bioavailability (narrow therapeutic index), biosimilar medicines, and multiple ingredients products.
Your drug prescriber will decide if you are to stick with a particular brand, or you can substitute it with a generic drug.
Before we understand the differences between branded and generic medicines, we will explain each of them.
Branded Medicine (Brand Name Drugs, Proprietary Medicine)
A branded medicine is an original drug prepared by a pharmaceutical company. They hold the patent over the product.
When a pharmaceutical company discovers and develops a new drug (also called pioneer drugs, or innovator drug) they file for a patent to prevent other companies from making copies of the medication for a given period.
At this stage, the medicine has two names, the generic name (scientific name of the drug), or non-proprietary name and the brand name (also called proprietary name), which the company uses to market the product.
Branded drugs are costly because pharma companies spend a lot of money on research and development of new drug molecules, animal and human (clinical) research, as well as marketing.
Examples of branded medicines are:
| Brand names | Generic names |
| Tylenol® | Acetaminophen |
| Zoloft® | Sertraline HCI |
| Xanax® | Alprazolam |
| Lomotil® | Diphenoxylate hydrochloride/atropine sulfate |
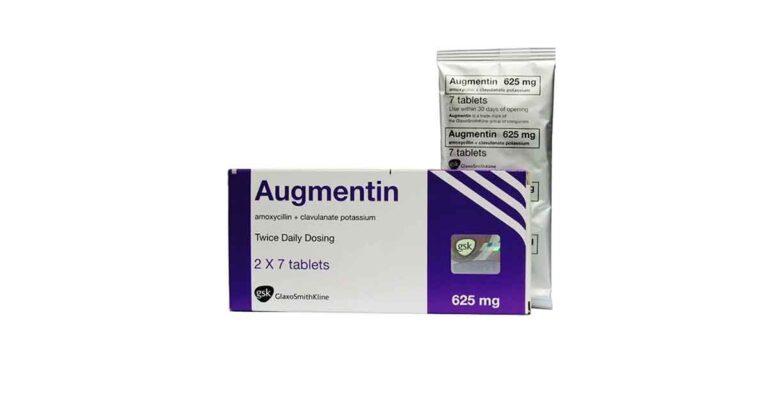
| Augmentin® | Amoxicillin and Clavulanate |
| Premarin® | Conjugated estrogens |
Generic Medicine (Non-Proprietary Medicine)
Most patents for branded medicines last up to 20 years of marketing protection. The company that produced the brand or a different company can now produce the generic version of the brand name drug.
A Generic medicine is similar to branded medicine in dose, strength and active pharmaceutical ingredients (API) but are marketed under different names by the companies.
According to the FDA, “A generic drug is a medication created to be the same as an already marketed brand-name drug in dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use”.
Through bioequivalence studies, generic medicines are compared with the innovator products (branded) to make sure they are therapeutically equivalent.
Bioequivalence is the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same dose
WHO (World Health Organisation)
Generic drugs are cost-saving for patients as they drive down prices owing to competition. Almost more than 50 percent of drugs prescribed in the hospitals today are generic medicines, and they cover different diseases such as diabetes, malaria, HIV, etc.
Differences Between Generic vs Brand Name Drugs
The major differences between generic vs. brand name drugs are:
- They must differ in shapes, sizes, flavouring, names and markings (according to Trademark laws) as these properties do not affect how the drugs work.
- The inactive ingredients might be different in generic vs brand name drugs. Some people might react to certain dyes used for drug production, hence some companies substitute it. However, the active ingredients in both branded and generic medicines must be the same.
- Different pharmaceuticals manufacture different versions of generic medicines.
- Generic medicines cost around 20 to 80 percent less than branded medicines.
Similarities Between Generic vs Brand Name Drugs
Though there are obvious differences between generic vs brand name drugs, there are some clear similarities. They are:
- Both generic and branded medicine have the same route of administration.
- The manufacturing process for both forms is the same. Strict compliance to standards such as purity, quality and strength is also the same.
- The drug information label is also the same.
- They are used for treating the same condition (the same indication)
- They have the same dosage strength, which is the amount of active ingredients (e.g. 20 mg, 60 mg)
- Both drugs must have the same active ingredients (the chemical substance that makes up the drug)
- Generic and brand drug medicines must be in the same dosage form, e.g. liquid, suspension, tablet.
- They should deliver a comparable amount of drug to the bloodstream within a given period.
Conclusion
Though there are common misconceptions about the effectiveness of branded over generic medicines, it is important to note that they are similar in effectiveness. A large-scale study showed a slight difference of around 3.5% between the absorption of generic and branded medicines. This is still within acceptable range.
Generic medicines are also safe to use. The use of low quality materials is not always the case, contrary to popular beliefs. We cannot rule out that some pharma manufacturers might carry out this unethical practice.